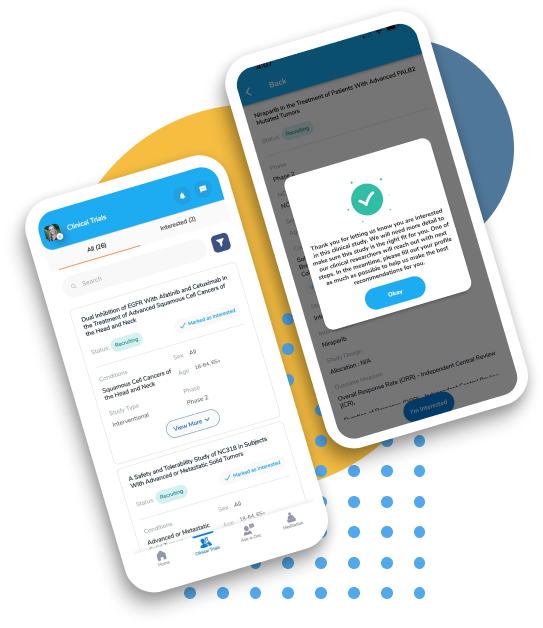
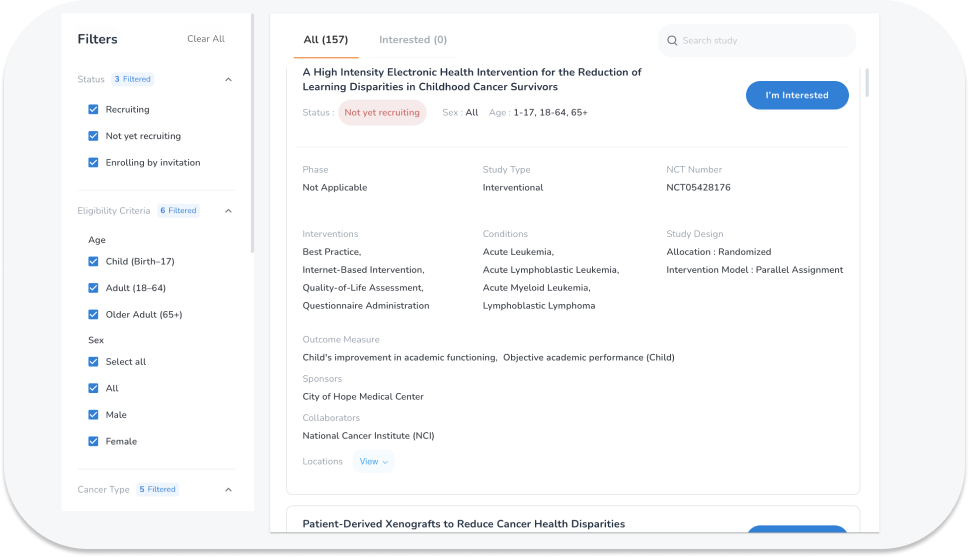
Let OncoPower help you beat cancer. Absolutely Free!
We help you find, explain treatments in clinical trials and connect with the investigator. Search, Compare and Get Personalized Results Based on Your Condition Right From Your Home! Find Clinical Trials Now.